Abstract
Introduction: Many patients (pts) with lower-risk myelodysplastic syndromes (LR-MDS) die without progressing to higher-risk (HR)-MDS or acute myeloid leukemia (AML), and the underlying causes are unclear. This analysis aimed to describe clinical characteristics, treatment patterns, outcomes, and causes of death (COD) of pts with LR-MDS in the Connect ® Myeloid Disease Registry (NCT01688011).
Methods: This US multicenter, prospective, observational study has 5 cohorts (idiopathic cytopenias of undetermined significance, LR-MDS, HR-MDS, AML, and myelofibrosis) and began enrollment in December 2013 (Steensma, BMC Cancer 2016;16:652). Pts in the LR-MDS cohort are ≥ 18 years old, newly diagnosed with Low or Int-1 risk MDS by IPSS, and stratified by baseline anemia as follows: no/mild (> 10 g/dL), moderate (8-10 g/dL), and severe (< 8 g/dL). Transfusion dependence was defined as ≥ 1 transfusion/8 weeks. First-line therapy categories were erythropoietin-stimulating agents (ESAs), hypomethylating agents (HMAs) and immunomodulatory imide (IMiD) agents.
Results: As of February 19, 2021, 531 pts with LR-MDS (mean age 74.0 years, 66.5% male) had enrolled; 215 pts (40.6%) were classified as Low risk, and 314 (59.2%) were classified as Int-1 risk by IPSS. At enrollment, 194 pts (36.5%) had no/mild, 232 (43.7%) had moderate, and 105 (19.8%) had severe anemia. At baseline, 112 pts (21.1%) were transfusion dependent.
Three hundred thirty pts (62.1%) received no treatment/supportive care at baseline, including 163 pts (49.4%) with no/mild anemia, 122 (37.0%) with moderate anemia, and 45 (13.6%) with severe anemia. Thirty-eight pts (11.5%) died without receiving any active treatment.
At baseline, 192 pts (36.2%) pts received an ESA, IMiD agent, or HMA as their initial treatment; 80 (15.1%) received ESAs, 23 (4.3%) received IMiD agents, and 89 (16.8%) received HMAs. Of 80 pts who received ESAs, 23 (28.8%) continued or started another ESA, 29 (36.3%) went on to receive no treatment/supportive care only, 10 (12.5%) received HMAs, 5 (6.3%) received IMiD agents, 1 (1.3%) discontinued therapy, and 9 (11.3%) died. Of 23 pts who received IMiD agents, 11 (47.8%) continued IMiD therapy, 4 (17.4%) went on to receive no treatment/supportive care only, 2 (8.7%) received HMAs, 3 (13.0%) discontinued therapy, and 3 (13.0%) died. Of 89 pts who received HMAs, 30 (33.7%) continued or started another HMA, 37 (41.6%) received no treatment/supportive care only, 1 (1.1%) started ESAs, 1 (1.1%) started IMiD agents, 1 (1.1%) progressed to AML, and 11 (12.4%) died.
Out of the 192 pts who received initial treatment, 30 (15.6%) had no/mild, 104 (54.2%) had moderate, and 58 (30.2%) had severe anemia at baseline. Of the 30 pts with no/mild anemia, 8 (26.7%) received ESAs, 20 (66.7%) HMAs, and 2 (6.7%) IMiD agents. Of the 104 pts with moderate anemia, 47 (45.2%) received ESAs, 46 (44.2%) HMAs, and 11 (10.6%) IMiD agents. Of the 58 pts with severe anemia, 25 (43.1%) received ESAs, 23 (39.7%) HMAs, and 10 (17.2%) IMiD agents.
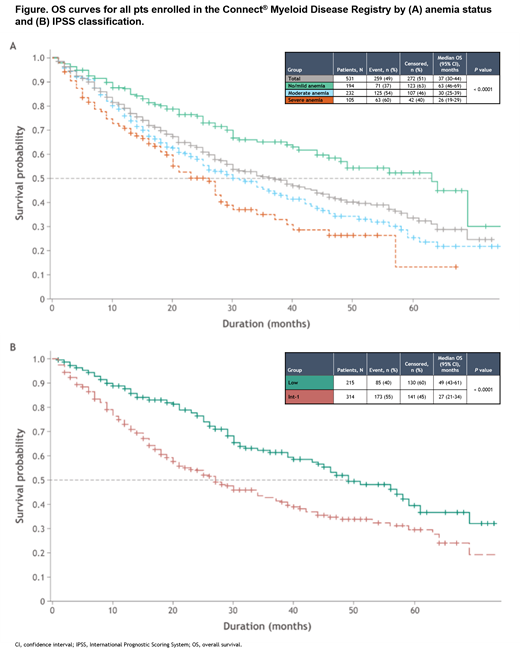
Median overall survival (OS) was 37 months for all pts; 63, 30, and 26 months for pts in the respective anemia groups (Figure A); and 49 months for Low risk and 27 months for Int-1-risk pts (Figure B). AML progression occurred in 35 pts (6.6%), including 13 (6.7%), 13 (5.6%), and 9 (8.6%) pts with no/mild, moderate, and severe anemia, respectively (P = 0.56). Death occurred in 213 pts (40.1%), including 60 (30.9%), 100 (43.1%), and 53 (50.5%) pts in the respective anemia groups (P = 0.0021); 117 (54.9%) deaths were MDS-related (disease progression, infection, and bleeding). Cardiovascular disease (CVD) was the COD of 27 pts (12.7%), with 7 (11.7%), 12 (12.0%), and 8 (15.1%) pts in the respective anemia groups. COD was other/unknown in 69 pts (32.4%). Ninety-three pts (17.5%) were lost to follow-up.
Conclusions: In this analysis, 63.5% pts with LR-MDS in the Registry were diagnosed with moderate or severe anemia; however, the majority received no treatment/supportive care only. Pts with moderate or severe anemia had significantly worse OS than pts with no/mild anemia. Although > 90% of pts did not progress beyond LR-MDS to date, the mortality of half of all pts was directly related to their disease. The interplay between the disease and other COD should be further explored. Understanding pt management in clinical practice may inform if earlier therapeutic intervention and/or novel therapies could improve pt survival and outcomes.
Komrokji: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Speakers Bureau; BMSCelgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Taiho Oncology: Membership on an entity's Board of Directors or advisory committees; Acceleron: Consultancy; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Geron: Consultancy. George: Bristol Meyers Squibb: Consultancy; Celgene: Consultancy; Blueprint Medicines: Consultancy; Incyte Corporation: Consultancy. Erba: AbbVie Inc; Agios Pharmaceuticals Inc; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Incyte Corporation; Jazz Pharmaceuticals Inc; Novartis: Speakers Bureau; AbbVie Inc; Agios Pharmaceuticals Inc; ALX Oncology; Amgen Inc; Daiichi Sankyo Inc; FORMA Therapeutics; Forty Seven Inc; Gilead Sciences Inc; GlycoMimetics Inc; ImmunoGen Inc; Jazz Pharmaceuticals Inc; MacroGenics Inc; Novartis; PTC Therapeutics: Research Funding; AbbVie Inc; Agios Pharmaceuticals Inc; Astellas; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Daiichi Sankyo Inc; Genentech, a member of the Roche Group; GlycoMimetics Inc; Incyte Corporation; Jazz Pharmaceuticals Inc; Kura Oncology; Nov: Other: Advisory Committee; AbbVie Inc: Other: Independent review committee. Scott: Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Grinblatt: Astellas Pharma, Inc.: Consultancy; Bristol Myers Squibb: Consultancy; Astra Zeneca: Consultancy; AbbVie: Consultancy. Maciejewski: Novartis: Consultancy; Regeneron: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Alexion: Consultancy. Roboz: Agios: Consultancy; Mesoblast: Consultancy; Glaxo SmithKline: Consultancy; Jazz: Consultancy; Otsuka: Consultancy; Novartis: Consultancy; Amgen: Consultancy; Helsinn: Consultancy; Astellas: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy; Blueprint Medicines: Consultancy; Daiichi Sankyo: Consultancy; Actinium: Consultancy; AbbVie: Consultancy; Janssen: Research Funding; Janssen: Consultancy; Astex: Consultancy; Bayer: Consultancy; MEI Pharma - IDMC Chair: Consultancy; Jasper Therapeutics: Consultancy; Pfizer: Consultancy; Bristol Myers Squibb: Consultancy; Roche/Genentech: Consultancy. Savona: AbbVie: Consultancy, Other: Travel expenses; Bristol Myers Squibb/Celgene: Consultancy, Other: Travel expenses; Geron: Consultancy; Incyte: Consultancy, Other: Travel expenses, Research Funding; Novartis: Consultancy; Ryvu Therapeutics: Consultancy, Other: Travel expenses; Sierra Oncology: Consultancy, Other: Travel expenses; Taiho Pharmaceutical: Consultancy; Takeda: Consultancy; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; ALX Oncology: Research Funding; Karyopharm Therapeutics: Current equity holder in publicly-traded company; Astex Pharmaceuticals: Research Funding. DeGutis: BMS: Current Employment, Current equity holder in publicly-traded company. Kiselev: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Yu: BMS: Current Employment. Makinde: Bristol Myers Squibb: Current Employment. Sekeres: Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal